RepliGut® Crypt
A self-renewing intestinal epithelium for evaluating long-term drug exposure and epithelium repair capability.
RepliGut® Crypt
RepliGut® Crypt is the first primary stem cell-derived platform that recapitulates cell turnover in the gut (proliferation, differentiation, and migration), enabling unparalleled ability to assess repair and renewal in a controllable in vitro model system.
Using engineered cell culture inserts, primary intestinal cells establish a 2D self-organized tissue spatially segregated into a stem cell niche alongside proliferative, differentiating, and mature cells of absorptive and secretory lineages. When the established epithelium in RepliGut® Crypt is damaged through chemical or inflammatory mechanisms, researchers can now study the repair, renewal, and regeneration effects of new therapeutics or damage mitigators in a human-relevant high-throughput system.
Key Features of RepliGut® Crypt systems
The standardized plate format with access to both apical and basal surfaces enables wide range of assays to suit your needs.
Click each button below to learn more
Mimicking the in vivo crypt
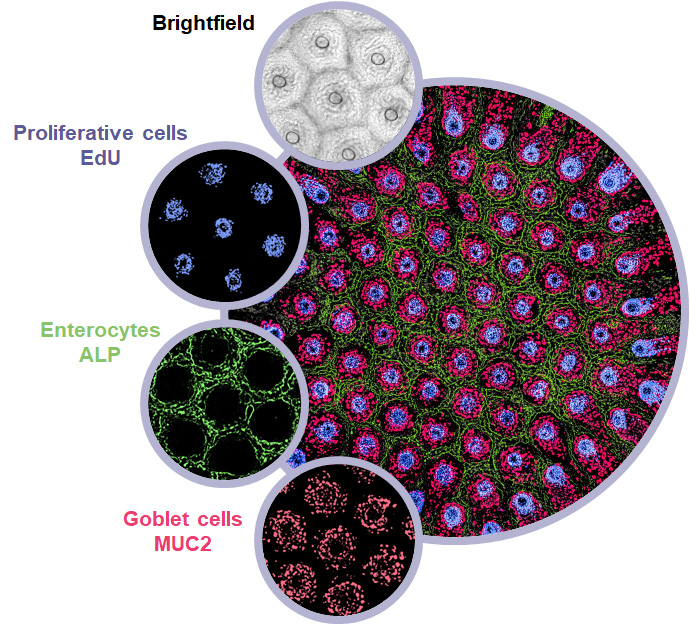
Unlike a standard Transwell membrane that is highly permeable, RepliGut® Crypt is cultured on an impermeable substrate laser drilled with 91 microholes arranged in a precise hexagonal array. Primary human epithelial stem cells derived from post-mortem colonic crypts are grown on hydrogel-coated inserts until cells reach confluence. Growth factors in the basal media produce a growth factor gradient within the hydrogel scaffold, promoting self-organized tissue development. Cells that reside over the microholes maintain a proliferative status (EdU+) while cells adjacent to the holes differentiate into secretory (MUC2+) and absorptive (ALP+) lineages.
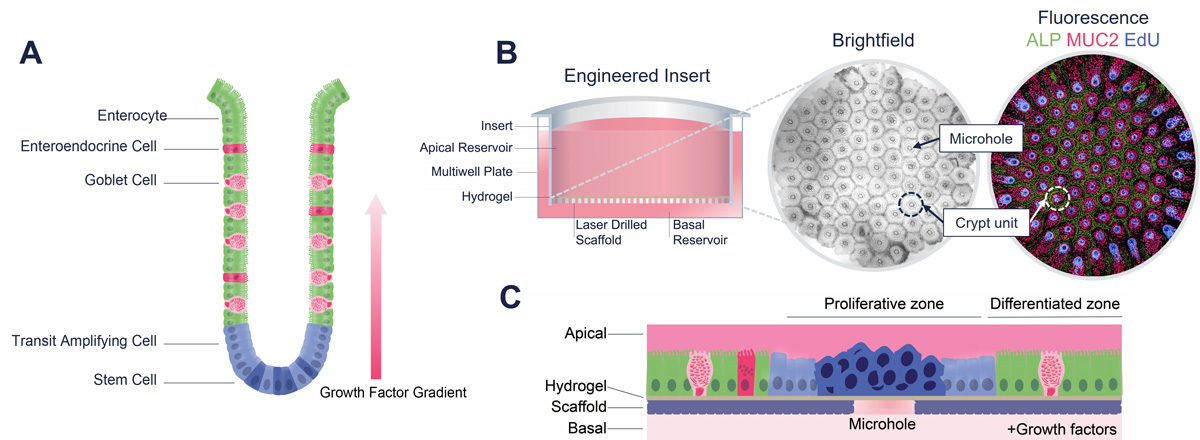
RepliGut® Crypt. (A) Localization of key cell types within the intestinal crypt. (B) Schematic of the engineered cell culture insert and crypt array comprised of 91 crypt units. Each crypt unit is centered around a microhole and contains an EdU+ proliferative zone surrounded by a MUC2+ and ALP+ positive differentiated zone. (C) Schematic of proliferative and differentiated zones.
Self-renewing capability
The self-renewing capability of RepliGut® Crypt recapitulates cell turnover in the gut, facilitating the investigation of epithelium damage and repair capabilities. EdU+ cells proliferate, migrate and differentiate into ALP+ and MUC2+ cell populations that are maintained for up to 14 days.

Pulse chase experiment reveals directional migration of cells along the crypt unit in RepliGut® Crypt. (A) After a 24-hour EdU pulse, the majority of EdU+ cells reside over the microholes. Radial migration of EdU labeled cells towards the border of crypt units is observed across 6 days of culture. (B) Migration of EdU+ cells was quantified by measuring the radius of the EdU+ zone over time. Four crypt units were measured per array. Data are represented as mean SD. N =3 crypt arrays.

Longitudinal analysis of cell populations in RepliGut® Crypt. (A) Timeline and representative images of the crypt unit throughout culture progression. (B) Cell type quantification over time. Data are represented as mean SD. N =3 crypt arrays.
Custom image analysis
Custom image analysis enables quantification of fluorescent % positive area for each crypt unit. Our standard staining panel includes DAPI (nuclei), EdU (proliferative nuclei), MUC2 (goblet cells/mucus), and ALP (mature enterocytes)

High content image analysis for detecting cell populations. (A) Brightfield image of the crypt array. Blue circles indicate individual crypt units. Red holes indicate the microholes. (B) Fluorescence micrograph of EdU+ cells. Purple circle indicates one crypt unit. (C) Quantification of EdU percent positive area. Each data point represents one crypt unit.
Applications
Our innovative platform is versatile, making it ideal for a range of applications, including:
Epithelial damage or repair capabilities
Inflammatory disease modeling
Stem Cell proliferation studies
Drug safety assessment
Drug efficacy and lead optimization
Assess ability to Stimulate Stem Cell Repopulation
The self-renewing capability of RepliGut® Crypt allows for assessment of pro- or anti-proliferative effects of compounds.

IL-22 stimulates proliferation of stem cells to promote renewal of epithelium. (A) EdU+ and (B) ALP percent positive area. A 7-day treatment with the cytokine IL-22 significantly reduced ALP+ enterocytes and increased EdU+ cells. These effects reversed after a 7-day washout. Each data point represents one crypt unit. Black line indicates mean. Significance was determined using a Kruskal-Wallis test. N =3 crypt arrays per time point.
Why choose RepliGut® Crypt?
Relevance to human biology
RepliGut® Crypt closely mimics the architecture of the human intestinal epithelium, offering better human relevance than standard cell lines.
Dynamic cell behavior
The juxtaposition of proliferative and differentiated cells enables the study of dynamic cellular behaviors, such as differentiation, migration, and response to stimuli.
Enhanced cell functionality
Combining stem and differentiated cells better represents the intestinal environment, facilitating the exploration of cellular interactions.
Capabilities Summary
Click each to read more
Apparent permeability (Papp) and efflux ratios
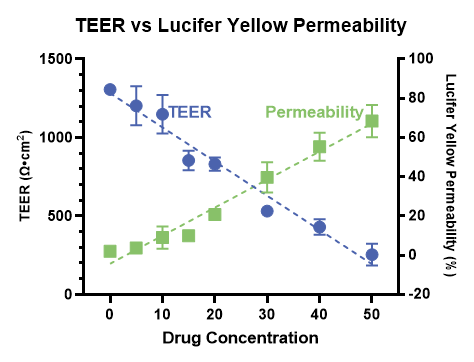
Human jejunum epithelial stem cells are cultured and differentiated on semi-permeable membrane inserts in a 96-well plate to facilitate access to both the apical and basal sides of the cell monolayer. Test compounds are introduced into either compartment to assess their transport in both directions. Accurate mass spectrometry analysis of compound concentrations in each compartment allows for the calculation of permeability (Papp) and efflux ratios. Controls such as atenolol and propranolol, representing low and high permeability respectively, can be included. Additionally, transporter inhibitors can be applied to assess compound interactions with specific transporters. These assays are adaptable to specific research needs.
Typical study design | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Cell Culture Timeline | 10-11 days | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Number of Replicates | Typically 3 wells per treatment | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Incubation Time | Up to 120 min | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Incubation Buffer | HBSS + 10mM HEPES + 10mM Glucose, pH 7.4 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Barrier Integrity Assessment | Automated TEER using EVOM™ Auto (WPI) measured before and after compound incubation | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Control Drugs | Atenolol and propranolol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Analysis Method | Accurate mass spectrometry measurement using LC/MS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Data Readout | Papp (apparent permeability coefficient), Efflux ratio, TEER | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
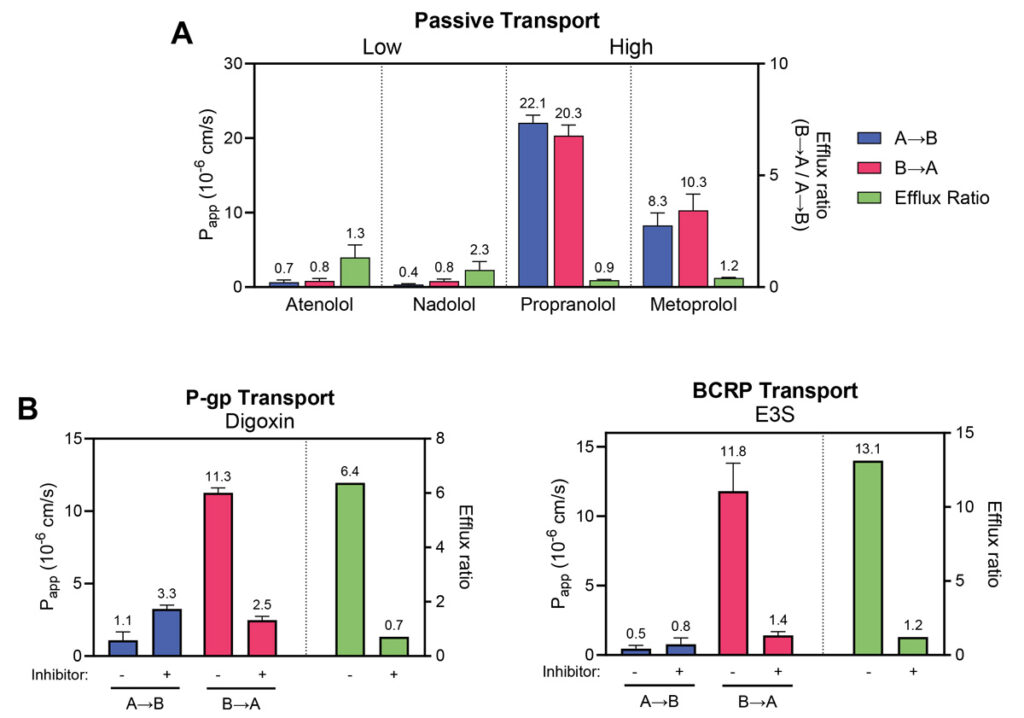
Permeability markers tested in RepliGut® Planar-Jejunum
Absorption Group | Drug | RepliGut® A→B Papp (10-6 cm/s) | Human Fraction Absorbed (Fa) |
|---|---|---|---|
High absorption (Fa ≥ 85%) | Antipyrine | 14.7 ±2.3 | 97% |
Metoprolol | 10.5 ±5.3 | 95% | |
Propranolol | 22.1 ±1.0 | 90% | |
Carbamazepine | 12.8 ±3.2 | 85% | |
Moderate absorption (Fa = 50 – 84%) | Ranitidine | 0.98 ±0.2 | 50% |
Atenolol | 0.97 ±0.9 | 50% | |
Low absorption (Fa < 50%) | Nadolol | 0.47 ±0.29 | 34% |
Lisinopril | 0.17 ±0.14 | 25% |
Absorption Group | Drug | Transporter | Efflux Ratio |
|---|---|---|---|
Efflux Substrates | Antipyrine | P-gp | 12.5 ±5.8 |
Estrone-3-sulphate | BCRP | 30.7 ±14.4 |
Intestinal contribution to metabolic stability
Human jejunum epithelial stem cells are cultured and differentiated on semi-permeable membrane inserts in a 96-well plate to facilitate access to both the apical and basal sides of the cell monolayer. Test compounds are introduced into either compartment to assess their transport in both directions. Accurate mass spectrometry analysis of compound concentrations in each compartment allows for the calculation of permeability (Papp) and efflux ratios. Controls such as atenolol and propranolol, representing low and high permeability respectively, can be included. Additionally, transporter inhibitors can be applied to assess compound interactions with specific transporters. These assays are adaptable to specific research needs.
Drug metabolism and transporter gene expression analysis
Transcriptomic analysis of RepliGut® Planar Systems can provide insights into gut-specific metabolic activity and potential for drug-drug interactions in the GI tract. We offer QuantiGene™ Plex Gene Expression Assays or TaqMan probe RT-PCR to provide targeted gene expression analysis to focus on the impact to your genes of interest. A reference RNAseq data set is available to confirm expression of your gene of interest in RepliGut® Planar- Jejunum.

Accurate mass spectrometry method development with quantitative or qualitative bioanalysis
Altis has partnered with BioAgilytix Labs to offer method development and bioanalysis using state of the art LC/MS instrumentation including a high-throughput SCIEX Echo® MS+ coupled to a SCIEX ZenoTOF 7600.
Comparisons between intestinal regions and donors
We have isolated epithelial stem cells from multiple intestinal regions obtained from multiple donors, allowing researchers to study colon or small intestine-specific metabolism and transport in demographically diverse backgrounds (e.g. age, sex, blood type). Our cryopreserved biobank enables the ability to work with the same donor as your project progresses or have a variety of donors to evaluate donor differences.

Companion barrier integrity monitoring
Accurate measurement of paracellular transport requires a consistently tight and intact cell culture barrier throughout the entire experiment. RepliGut® Planar barrier integrity is monitored in real time using Transepithelial Electrical Resistance (TEER). TEER is measured using the high-throughput and automated EVOM™ Auto (WPI) before and after compound incubation to ensure barrier integrity is not compromised.