Custom Services
Custom RepliGut® Services. Derived from human donor intestinal tissue, with a standardized workflow for reliable and reproducible data, RepliGut® Systems represent the next generation of in vitro models delivering faster actionable data.
Supportive, Consultative Approach
Efficient and effective project design guided by project leads with hands-on experience.
Full visibility to ongoing work with frequent communication.
High success rates with reliable timelines and consultation throughout.
Supportive environment of collaboration, a key ingredient to driving valuable, actionable insight.

Custom RepliGut® Services
Committed to delivering exactly what you need, we work closely with you to design the perfect study. For every custom project, our project leads help you choose your region of interest, explore our donor biobank, and decide on the important outputs.
Common Project Types
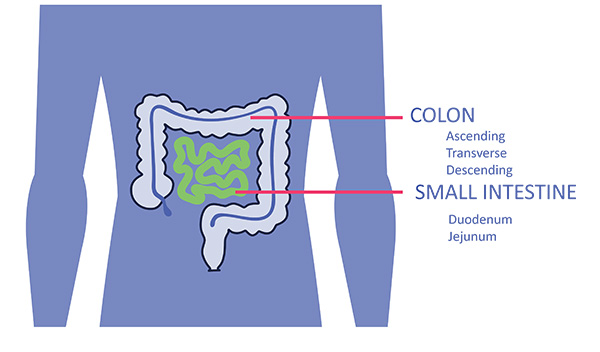
Unlike spherical organoids, RepliGut® Planar cultures directly enable access to both apical and basal surfaces independently. The models are sequentially stimulated to proliferate then differentiate which further enriches understanding of cell type involvement in many different application areas
Intestinal Barrier Function
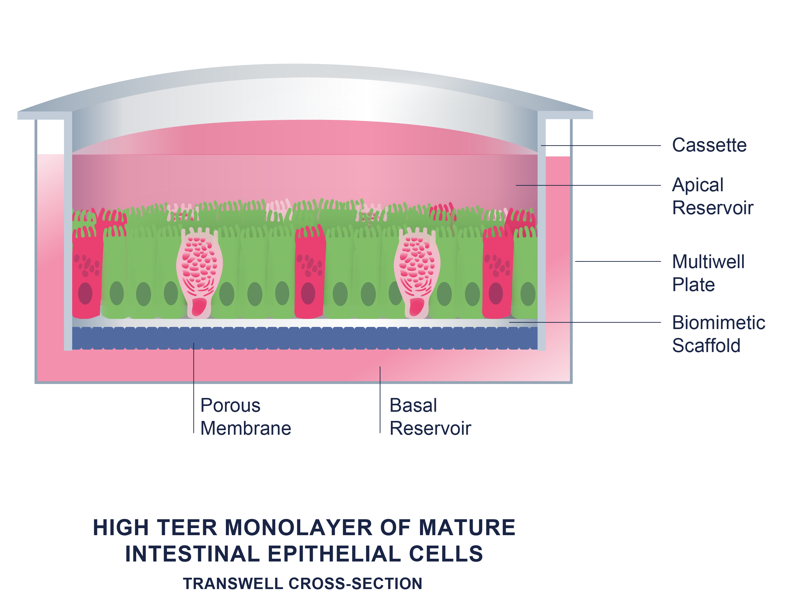
Integrity of the intestinal epithelium is crucial for the normal functioning of the lower GI tract. RepliGut® Models maintain barrier integrity with high TEER values. Compounds can be assessed for the potential to disrupt barrier function.
Inflammatory Response
Inflammation of the GI tract is the main hallmark of Inflammatory Bowel Disease. RepliGut® Models are comprised of polarized intestinal epithelial cells grown on coated semi-permeable membrane supports. Dosing and sampling of inflammatory cytokines can be performed in either the apical or basal compartments.
GI Toxicity
GI toxicities are the most common adverse events during Phase 1 clinical trials. RepliGut® Models recapitulate intestinal epithelium and can be used to screen compounds for drug-induced GI toxicity early in the drug development process.
Intestinal Drug Disposition
RepliGut® offers a remarkable capability to assess the absorption or release of compounds, in a format similar to Caco-2 cells. What sets RepliGut® Models apart is the ability to perform studies with cells derived from various regions of the small intestine and colon. The versatility of RepliGut® System enables testing with multiple donors, expanding the possibilities for comprehensive analysis.
Available Biological Endpoints
RepliGut® Planar cultures consist of a monolayer of differentiated intestinal cells with all the post mitotic lineages found in vivo forming a tight barrier system. The easy to grow and use format is highly amenable to studying standardized biological endpoints.
- Transepithelial electrical resistance (TEER)
- Permeability (Papp or % of control)
- mRNA Expression (RT-PCR)
- Cytotoxicity (ATP, LDH)
- Protein Expression (ELISA or Luminex)
- Immunocytochemisty
- High Content Imaging
Ready to Go With Your Gut?
Contact us to learn more about RepliGut® Model and Services.

