RepliGut® StemTox™ Services
Why GI toxicity is important
Gastrointestinal (GI) toxicity is the most prevalent adverse event associated with either orally or systemically administered marketed therapeutics. Drugs that cause GI toxicity are structurally and mechanistically diverse, often impacting barrier functions of the epithelium at the proliferative stem and progenitor cell level. When GI epithelia lose integrity, a broad range of clinical symptoms are likely to emerge, including diarrhea, inflammation, and increased probability of infection. These liabilities are often not discovered until late in pre-clinical development, if at all.


RepliGut® StemTox™ Services
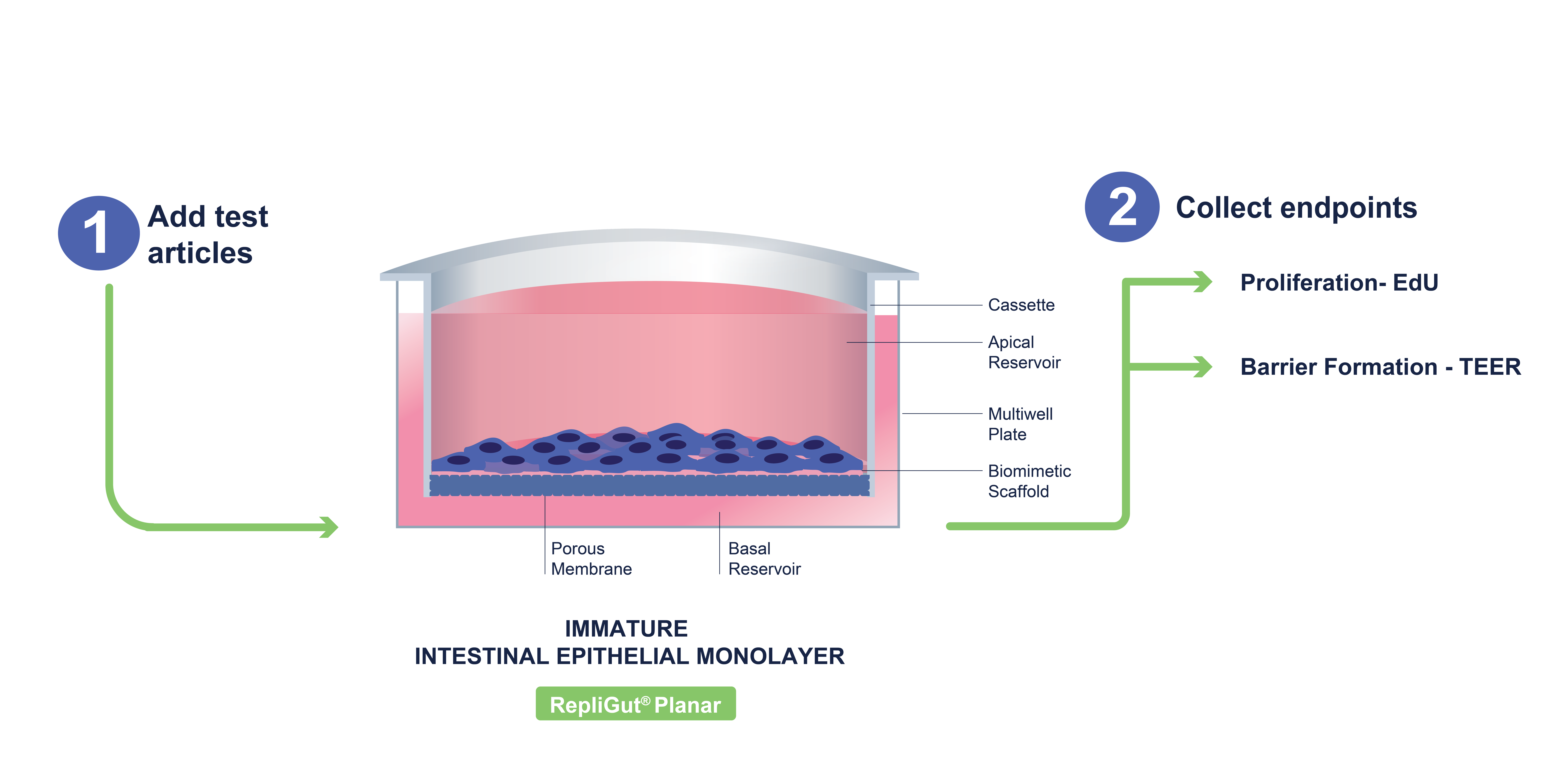
Our RepliGut® StemTox™ Services consists of a suite of human intestinal stem cell-based assays that has been optimized using RepliGut® Planar for sensitive and selective investigation of a drug or toxicant to disrupt cell proliferation and subsequent formation of the gut epithelial barrier. The StemTox™ Assay design uses a 96-well format and allows for high throughput assessment of cell renewal capacity and barrier formation simultaneously in the same well, without long culture setup times or complicated fluidics.
With StemTox™ Services, you can:
Predict risk of intestinal epithelial side effects earlier in development.
Expect exceptional reproducibility and data quality.
Leverage a simplified study design to get what you need, fast.
Worried about on target/off tissue side effects in the intestine?
Find out whether your target is expressed in RepliGut® Planar models. Our data mining experts can query our proprietary bulk mRNA sequencing data sets of RepliGut Planar – Jejunum at 2, 4, 6, and 8 days in culture or in response to various metabolism gene expression inducers.
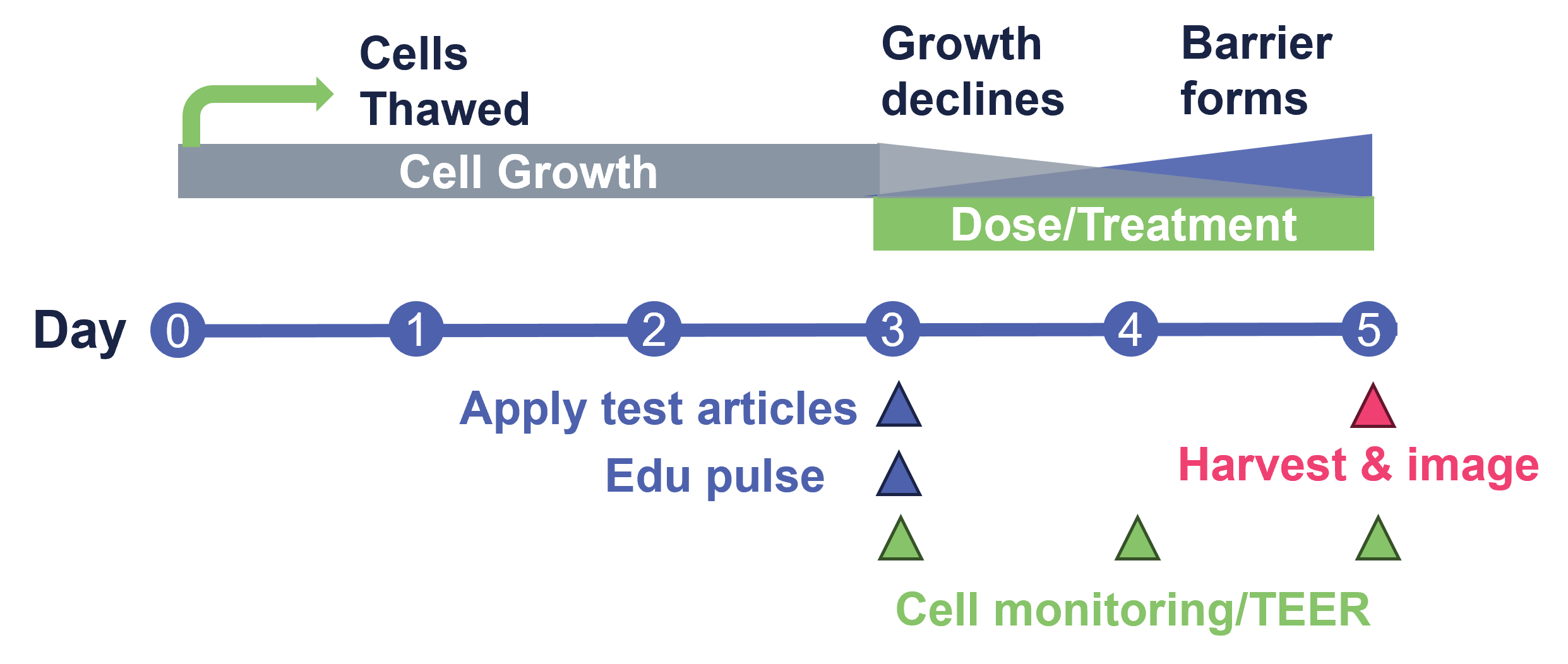
StemTox™ Assay Delivers Multiple Informative Endpoints From A Single 96-Well Plate In A Short 5-Day Assay.
We Quantify Results For:
Epithelial Barrier Formation - TEER
Trans-epithelial electrical resistance measurements to assess the ability for form a barrier
Barrier Renewal Capacity– EdU + DAPI cell counts
High-content imaging & image quantification for total cell count (DAPI+) and proliferating cell count (EdU+)
Figure 1 Overview of the StemTox™ Assay timeline from thawing of cells to final TEER and imaging results.
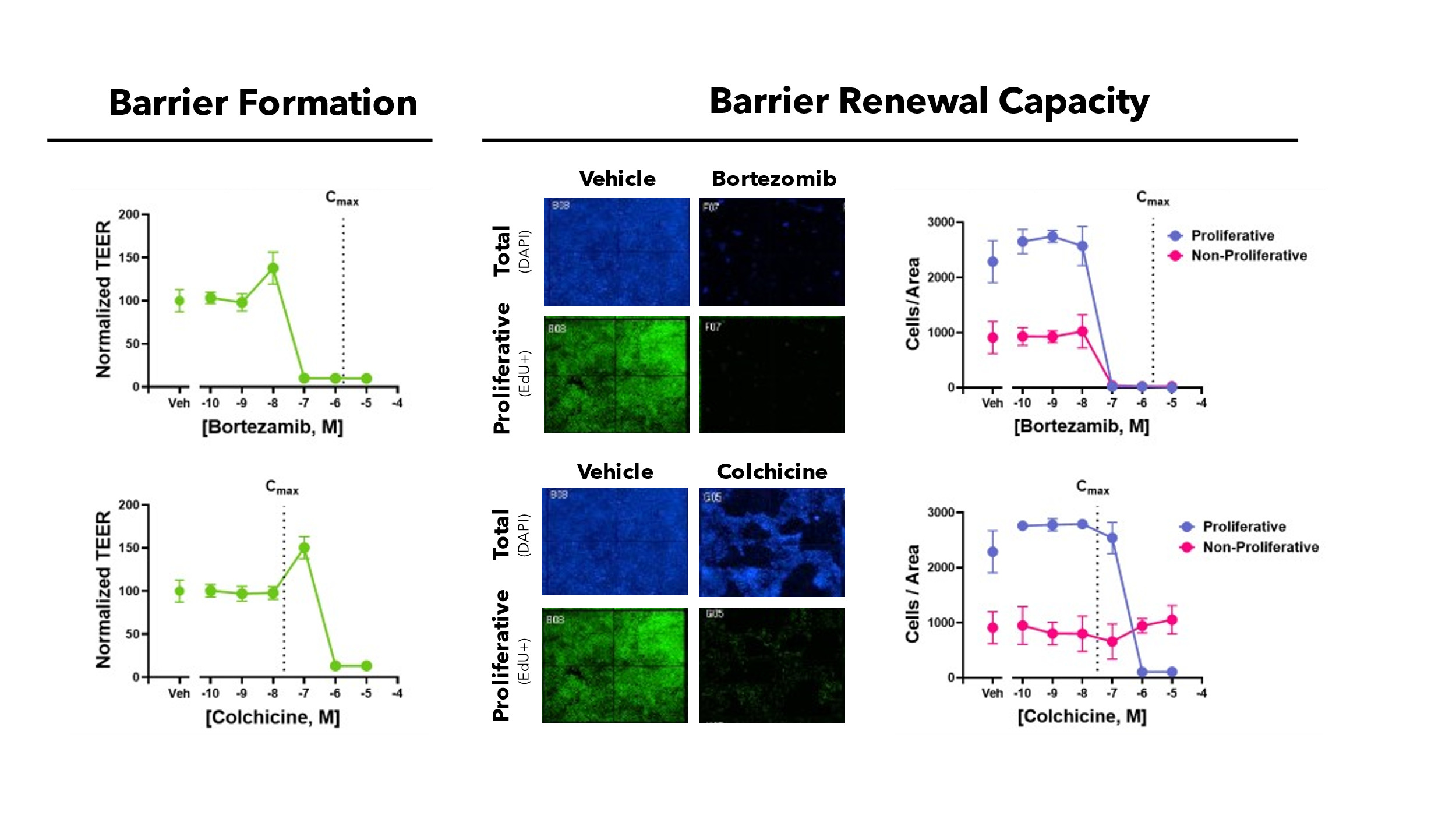
StemTox™ Assay Enables Query Of Barrier Formation And Renewal Capacity Simultaneously
Figure 2. Bortezomib and Colchicine are known to have significant GI side effects in the clinic. StemTox™ Assay is not only capable of detecting this toxicity (by measuring barrier formation and quantification of cell numbers) within 2 logs of the clinical Cmax, but was also able to show that Colchicine toxicity was limited to proliferating cells, whereas Bortezomib acted to disrupt GI epithelium in non-specific way.
Possible Add-On Studies Include
Gene Expression
Protein Expression
Immunohistochemistry
Broader range of endpoints in a faster timeline than organoids
Organoids in spherical form are inherently variable and are not amenable to many of the key endpoints, including high content imaging and barrier function assays. To utilize organoids in monolayer format for barrier studies requires first culturing the organoids over several weeks with two distinct culture expansions prior to achieving the monolayer. Using RepliGut® Planar, featuring direct to membrane cell culture, we can query both barrier formation and renewal capacity in a short 5-day timeline.
3D Organoids | RepliGut® Planar | |
|---|---|---|
Cytotoxicity | ||
Gene and Protein Expression | ||
Permeability | ||
High Content Imaging | ||
Planar monolayer | ||
Barrier integrity | ||
Barrier formation | ||
Barrier renewal capacity |
3D Organoids | RepliGut® StemTox | |
|---|---|---|
Cytotoxicity | Check | Check |
Gene and Protein Expression | Check | Check |
Permeability | Check | |
High Content Imaging | Check | |
Planar monolayer | Check | |
Barrier integrity | Check | |
Barrier formation | Check | |
Barrier renewal capacity | Check |
Get in touch with us
Have a question?
Want to learn more about RepliGut® Systems? Send us a Message